DiaClon ABD-Confirmation for Patients
ID-Card for the ABO/RhD blood group control of patients
Description
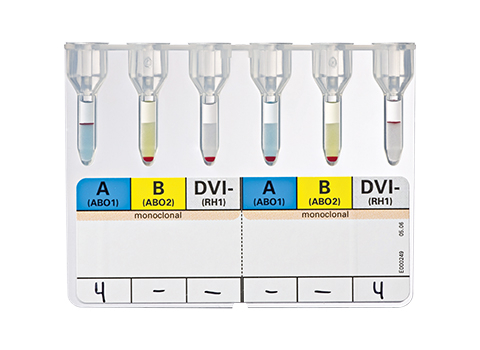
The DiaClon ABD patient confirmation ID-Card can be used for the ABO/RhD blood group control of patients. Current recommendations for RhD typing suggest that, for transfusion recipients and antenatal patients, anti-D reagents should not detect the DVI phenotype. Individuals possessing the DVI phenotype may produce an anti-D to the missing epitopes after immunization by fetal or transfused RhD positive cells. To ensure that appropriate therapeutic measures are instigated, a DVI patient's red cells should be assigned RhD negative status. Conversely, donor bloods should be tested with anti-D that does detect DVI and assigned RhD positive status, to avoid the unit being transfused to an RhD negative or partial-D patient.
CHARACTERISTICS
DiaClon ABD-Confirmation for Patients
50053
A, B, DVI-, A, B, DVI-
A: LM297/628 (LA-2)
B: LM306/686 (LB-2)
D: TH28, RUM-1, LDM1
| Catalog # (REF) | Description | Type of product |
|---|---|---|
| 001254 | A, B, DVI-, A, B, DVI- (ld-n°: 50053) 96 profiles, 4 x 12 | Reagents |
| 001257 | A, B, DVI-, A, B, DVI- (ld-n°: 50053) 576 profiles, 24 x 12 | Reagents |
| 001256 | A, B, DVI-, A, B, DVI- (ld-n°: 50053) 1,440 profiles, 60 x 12 | Reagents |
| 001255 | A, B, DVI-, A, B, DVI- (ld-n°: 50053) 2,688 profiles, 112 x 12 | Reagents |
To order or for more information, contact your local sales representative.
Find all our sales representatives on Bio-Rad.com